Overview

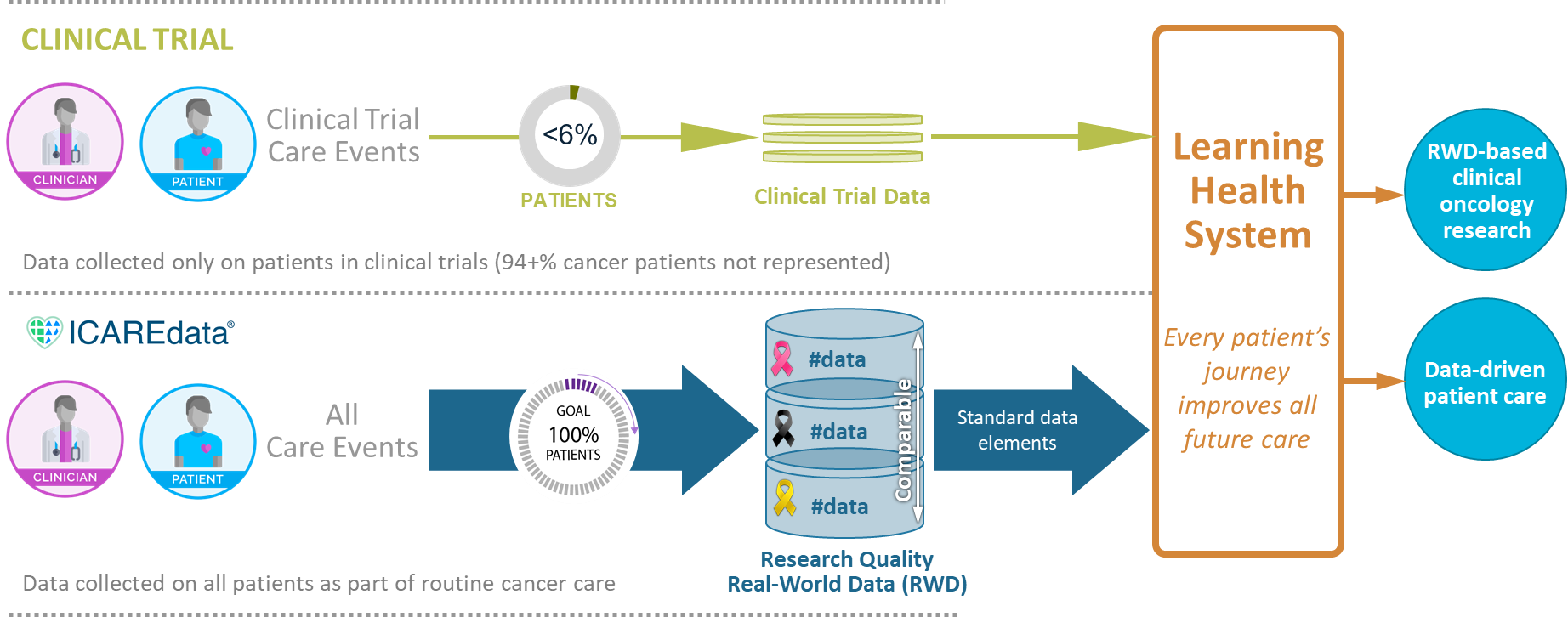
The overall goal of the ICAREdata® project is to enable clinical oncology research by prospectively gathering high quality real-world data (RWD). The ICAREdata project is a collaboration between MITRE, and the Alliance for Clinical Trials in Oncology and is being conducted in assocation with randomized controlled trials (RCT) and National Cancer Trials Network (NCTN) institutions. As a pilot use case for mCODE™ (Minimal Common Oncology Data Elements), the study will evaluate the outcome elements of mCODE and demonstrate a real-world data strategy for clinical trials based on mCODE. As part of the study, an open source infrastructure and automated standards-based interface for data capture and exchange are being developed and piloted.
Goals
Support the collection of high quality real-world data, based on mCODE, to
enable clinical oncology research
Increase data pool (RCT data + RWD)
Establishing large scale, prospective collection and integration of high quality clinical data across multiple clinical care sites to augment data collected from clinical trials.
Inform therapeutics development
Developing effective treatments for patients with rare tumors or uncommon disease types and understanding the natural history and response to therapy for diseases where multiple sequential therapeutic regimens are employed.
Inform regulatory decision-making
Helping to determine how to best use real-world evidence for medical product regulatory decision-making and achieving efficient and accurate post-market surveillance of FDA-approved therapeutics.
Inform data-driven patient care
Understanding efficacy and safety of approved therapeutic agents in underrepresented and minority populations and accumulating a large number of patients and data required to achieve success in personalized medicine.
Vision
Contact Us
ICAREdata Team
icaredata@alliancefoundationtrials.org

 ICAREdata® Project
ICAREdata® Project


